New drug delivery system could improve glaucoma treatment
Researchers from the Institute of Biomedical Engineering and Chemical Engineering at the University of Toronto have developed a novel method to dramatically extend the duration of glaucoma treatment. By leveraging colloidal drug aggregates (CDAs)—once considered a nuisance in drug development—the team has created a technology capable of releasing medication in the eye for seven weeks with a single injection – a 200-fold increase over the current standard of care. This breakthrough has the potential to improve patient outcomes while reducing systemic side effects, addressing a critical unmet need in ophthalmic care.
These findings were published in a recent issue of the journal Advanced Materials.
"This research focuses on turning colloidal drug aggregates from a challenge in drug discovery into an innovative solution for long-acting ocular drug delivery," says Dr. Mickael Dang, a recent PhD graduate and lead author of the study. "Specifically, we developed a technology that significantly extends the duration of glaucoma treatment while minimizing systemic side effects."
Glaucoma, a leading cause of irreversible blindness, is currently managed with eye drops that need to be applied multiple times a day. This frequent dosing is inconvenient for patients and results in poor adherence. As 95% of the eye drop drips down one’s cheek and what is left is rapidly cleared from the eye, the eye drops have limited efficacy and cause systemic absorption that can lead to undesirable side effects.
The new study addresses these issues by reimagining the role of colloidal drug aggregates, transforming them into a tool for sustained drug delivery.
"We achieved two firsts with this research," said Professor Molly Shoichet, the study's corresponding author. "We demonstrated that a soluble drug could be converted into a colloidal drug aggregate. While this isn’t typically desired, it allowed us to control the drug’s release. Second, we showed that this formulation could be injected non-invasively and effectively lower eye pressure in a rat model—a key aspect of glaucoma treatment—for seven weeks instead of the usual six hours. That’s 200 times longer!"
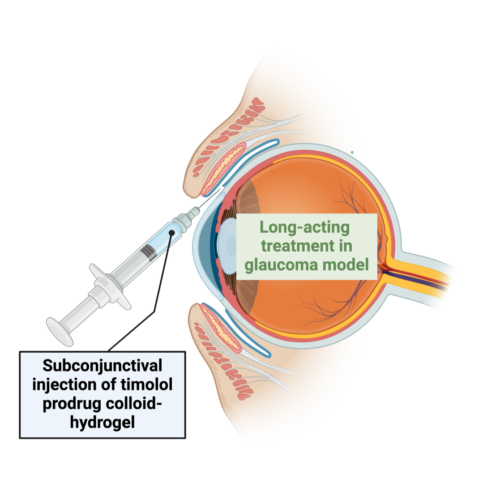
The team achieved this by chemically modifying a commonly used glaucoma drug, timolol, to form colloids. These colloids were embedded in a biocompatible hydrogel, which was injected into the subconjunctival space of rat eyes. The formulation allowed for the sustained release of timolol, reducing intraocular pressure over an extended period while maintaining low systemic drug concentrations. This minimizes the risk of side effects such as bradycardia, which can result from conventional eye drops.
"The ability to deliver glaucoma medication for such an extended period with a single, minimally invasive injection represents a significant advancement in ocular drug delivery," said Dang. "This approach could potentially improve patient compliance and treatment outcomes while reducing the need for frequent dosing."
This study was done in collaboration with Dr. Jeremy Sivak from University Health Network (UHN), who provided valuable insights. His expertise in glaucoma research was instrumental in shaping the study's clinical impact.
Looking ahead, Professor Shoichet noted “We’re excited for the future – we plan to advance this technology out of the lab and towards the clinic, ultimately impacting clinical care. We welcome partnerships on this journey.”